A375-Fluc-Neo/eGFP-Puro
- Frozen / Standard (CL085-STAN) $ 2,100
Species: Human
Cell type: Melanoma
Transgenes: Firefly luciferase (Fluc) with neomycin resistance (Neo) for selection with G418 and enhanced green fluorescent protein (eGFP) with puromycin resistance (Puro) for selection with puromycin
Media: DMEM, 10% FBS, 1% Pen/Strep, 0.6 mg/mL G418, 1 μg/mL puromycin
Description: A375-Fluc-Neo/eGFP-Puro is a polyclonal population of the human malignant melanoma cell line A375 (ATCC® CRL-1619™) transduced with LV-Fluc-P2A-Neo (LV011) encoding the firefly luciferase (Fluc) cDNA under the spleen focus-forming virus (SFFV) promoter linked to the neomycin resistance gene (Neo) via a P2A cleavage peptide and LV-eGFP-PGK-Puro (LV031) encoding the enhanced green fluorescent protein (eGFP) cDNA under the spleen focus-forming virus (SFFV) promoter and the puromycin resistance gene (Puro) under the mouse phosphoglycerate kinase (PGK) promoter.
The lentiviral vector used is a self-inactivating (SIN) vector in which the viral enhancer and promoter has been deleted. Transcription inactivation of the LTR in the SIN provirus increases biosafety by preventing mobilization by replication competent viruses and enables regulated expression of the genes from the internal promoters without cis-acting effects of the LTR (Miyoshi et al., J Virol. 1998).
Mycoplasma Testing: The A375-Fluc-Neo/eGFP-Puro cell line has been tested for mycoplasma contamination and is certified mycoplasma free.
Cell Line Authentication: The parental A375 cell line was authenticated are certified free of interspecies cross-contamination by short tandem repeat (STR) profiling with 9 STR loci including CSF1PO, D13S317, D16S539, D5S818, D7S820, TH01, TPOX, vWA and sex chromosome marker Amelogenin.
Recommended uses: In vitro: This is a high Fluc/eGFP expressing cell line suitable for use as a positive control cell line in bioluminescence and fluorescence assays to verify luciferase or GFP expression respectively in your lentiviral transduced cells. In vivo: A375 cells form tumors post implantation into immunosuppressed mice. The in vivo growth of these tumors can be monitored using noninvasive bioluminescent imaging.
Note: In-life imaging for GFP fluorescence is not recommended due to high background autofluorescence and low light penetration. Tissues may be harvested post mortem for analysis by conventional microscopy.
Publications that used associated reagents:
LV-eGFP-PGK-Puro (LV031): Shen et al. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood. 2016. March 17: 127(11): 1449-58.
Cell photos: Low- and high-density cell morphology (200X)

Flow Cytometry for eGFP: A375-Fluc-Neo/eGFP-Puro (green) or isotype control (A375-Fluc-Puro; grey) cells were fixed with paraformaldehyde and analyzed by flow cytometry (20,000 events).

Luciferase Assay: 104, 105, or 106 cells were placed in wells of a 96-well plate and 30 μg/mL of d-luciferin was added to the indicated wells. The plate was immediately imaged using a Xenogen IVIS Spectrum.

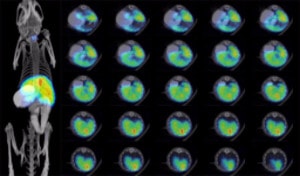
Bioluminescent images showing growth of a subcutaneous A375-Fluc tumor in an athymic mouse

107 A375-Fluc-Puro cells (Imanis Life catalog #CL055) were injected subcutaneously into the right flanks of female NCR athymic mice. Mice were imaged at day 0 on the day of cell implantation and subsequently at days 7 and 16 using a Perkin Elmer IVIS® Spectrum system, at 10-15 minutes post intraperitoneal injection of D-luciferin at 150 mg/kg. Tumor size was measured using calipers. Data from a representative mouse (ID#1299) is shown.