VV(Li)-RlucGFP-hNIS-tRFP
- 0.25 mL / Standard / Y ((OV4001-STAN)) $ 1,500
Ready to use vaccinia virus expressing Ruc-GFP, hNIS, and tRFP.
- Description: Replication competent vaccinia virus encoding Renilla luciferase (Ruc)-green fluorescent protein (GFP) fusion, human sodium iodide symporter (hNIS), and TurboFP635-like protein (tRFP). These additional transcription units are inserted into, and disrupt the function of, the viral F14.5L, J2R (thymidine kinase) and A56R (hemagglutinin) genes, respectively (see diagram below).

- Strain: Lister strain
- Transgenes: Green fluorescent protein (GFP), human sodium iodide symporter (hNIS), TurboFP635-like protein (tRFP), and Renilla luciferase (Ruc).
- Biosafety Level: 2
- Titer: Standard (clarified supernatant, at least 5e6 TCID50/ml).
- Propagation method: Inoculate producer cells (e.g. Vero) at an MOI of 0.1 for 2-3 hours. After 48 hours harvest culture supernatant and cells and freeze-thaw or sonicate 3X. Clarify the supernatant by low-speed centrifugation and purify through a sucrose cushion. Resuspend the viral pellet in 1mM TrisCl, pH 9.
- Proposed Use: Ready to use viral stock for propagation and cell killing assays.
- USDA permit required for shipping? Yes. Click here to fill out Application Form VS 16-3 to obtain a USDA APHIS VS 16-6 or 16-6A permit.
Transgene Validation
Fluorescence: Vero cells were mock-infected (A-C) or infected with VV(Li)RucGFP-hNIS-tRFP MOI 0.1 (D-F) and imaged at 48 h.p.i.

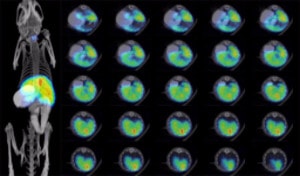
NIS Function: Vero cells were mock-infected or infected with VV(Li)-RucGFP-hNIS-tRFP at the indicated MOI. After 24 hours, uptake of 125I was measured in the presence or absence of KClO4, an inhibitor of NIS-mediated 125I uptake.

Luciferase Assay: Vero cells were mock-infected or infected with VV(Li)-RucGFP-hNIS-tRFP at an MOI of 0.1 or 1. After 48 hours, 1.25 µg of coelenterazine was added to each of the wells and luminescence (RLU) was immediately measured using a microplate reader.